Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 in NaCl
in NaClAoyama, Takahito; Ogawa, Hiroaki; Kato, Chiaki; Ueno, Fumiyoshi
Metals, 11(3), p.511_1 - 511_13, 2021/03
Times Cited Count:3 Percentile:24.75(Materials Science, Multidisciplinary)The effect of Cu in bulk solution on pitting corrosion resistance of extra high purity type 316 stainless steel was investigated. Pitting occurred in 0.1 M NaCl-1 mM CuCl
in bulk solution on pitting corrosion resistance of extra high purity type 316 stainless steel was investigated. Pitting occurred in 0.1 M NaCl-1 mM CuCl whereas pitting was not initiated in 0.1 M NaCl. Although deposition of Cu
whereas pitting was not initiated in 0.1 M NaCl. Although deposition of Cu on the surface occurred regardless of potential region in 0.1 M NaCl-1 mM CuCl
on the surface occurred regardless of potential region in 0.1 M NaCl-1 mM CuCl , Cu
, Cu in bulk solution had no influence on the passive film formation. The decrease in pitting corrosion resistance in 0.1 M NaCl-1 mM CuCl
in bulk solution had no influence on the passive film formation. The decrease in pitting corrosion resistance in 0.1 M NaCl-1 mM CuCl resulted from the deposited Cu or Cu compound and continuous supply of Cu
resulted from the deposited Cu or Cu compound and continuous supply of Cu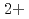 on the surface.
on the surface.
Yamamoto, Masahiro
Zairyo To Kankyo 2020 Koenshu (CD-ROM), p.9 - 16, 2020/05
The author has been continuing research and development for corrosion science for about forty years. One of the main targets of his research is applying computational science techniques on corrosion problems. The results are briefly introduced in this article. Also, the author organized some workshop for corrosion problems of 1F decommissioning procedure for several years. Such activities are evaluated for receiving the society award in JSCE.

 generation; The Role of NO
generation; The Role of NO
 in the crevice corrosion repassivation of type 316L stainless steel
in the crevice corrosion repassivation of type 316L stainless steelAoyama, Takahito; Sugawara, Yu*; Muto, Izumi*; Hara, Nobuyoshi*
Journal of the Electrochemical Society, 166(10), p.C250 - C260, 2019/01
Times Cited Count:5 Percentile:16.76(Electrochemistry)The role of NO
 in the repassivation of crevice corrosion of Type 316L stainless steel was investigated. In crevice corrosion tests, the solution was changed from 1 M NaCl to NaCl-NaNO
in the repassivation of crevice corrosion of Type 316L stainless steel was investigated. In crevice corrosion tests, the solution was changed from 1 M NaCl to NaCl-NaNO . NO
. NO
 led to complete repassivation. Repassivation of the crevice corrosion was found to take place in two steps. In the first step, the estimated current density inside the crevice gradually decreased from ca. 5 mA cm
led to complete repassivation. Repassivation of the crevice corrosion was found to take place in two steps. In the first step, the estimated current density inside the crevice gradually decreased from ca. 5 mA cm to ca. 5
to ca. 5  A cm
A cm . After that, the current density suddenly decreased to less than 0.1
. After that, the current density suddenly decreased to less than 0.1  A cm
A cm . From the potentiodynamic polarization in acidic solutions simulated inside the crevice (pH 0.2) and in situ observations of the crevice corrosion morphology, the first step was thought to be generated by the suppression of active dissolution by NO
. From the potentiodynamic polarization in acidic solutions simulated inside the crevice (pH 0.2) and in situ observations of the crevice corrosion morphology, the first step was thought to be generated by the suppression of active dissolution by NO
 . It would appear that the generation of NH
. It would appear that the generation of NH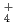 results in a pH increase and the further suppression of active dissolution, and then repassivation occurs.
results in a pH increase and the further suppression of active dissolution, and then repassivation occurs.
Taniguchi, Naoki; Kawakami, Susumu; *
JNC TN8400 2001-025, 27 Pages, 2002/03
It is essential to understand the corrosion type of carbon steel under the repository conditions for the lifetime assessment of carbon steel overpack used for geological isolation of high-level radioactive waste. According to the previous study, carbon steel is hard to passivate in buffer material assuming a chemical condition range of groundwater in Japan. However, concrete support will be constructed around the overpack in the case of repository in the soft rock system and groundwater having a higher pH may infiltrate to buffer material. There is a possibility that the corrosion type of carbon steel will be influenced by the rise of the pH in groundwater. In this study, anodic polarization experiments were performed to understand the passivation condition of carbon steel in buffer material saturated with water contacted with concrete. An ordinary concrete and a low-alkalinity concrete were used in the experiment. The results of the experiments showed that the carbon steel can passivate under the condition that water having pH  13 infiltrate to the buffer material assuming present property of buffer material. If the low-alkalinity concrete is selected as the support material, passivation can not occur on carbon steel overpack. The effect of the factors of buffer material such as dry density and mixing ratio of sand on the passivation of carbon steel was also studied. The results of the study showed that the present property of buffer material is enough to prevent passivation of carbon steel.
13 infiltrate to the buffer material assuming present property of buffer material. If the low-alkalinity concrete is selected as the support material, passivation can not occur on carbon steel overpack. The effect of the factors of buffer material such as dry density and mixing ratio of sand on the passivation of carbon steel was also studied. The results of the study showed that the present property of buffer material is enough to prevent passivation of carbon steel.
Akashi, Masatsune*; Fukaya, Yuichi*; Asano, Hidekazu*
JNC TJ8400 2000-015, 46 Pages, 2000/02
Difference of hydrogen generation phenomena on the surface of the Steels were not observed between carbon steel, atmospheric corrosion resisting steel and 5%-Ni steel. Rust layer was formed on these three-type of steels by steam oxidation method. And the chemical composition of the rust for the steels were basically two (2) layers structure for the previous two steels as hematite (Fe O
O ) based for the outer layer and magnetite (Fe
) based for the outer layer and magnetite (Fe O
O ) based for the inner layer. And for the last steel, it had three (3) layer in the rust as hematite (Fe
) based for the inner layer. And for the last steel, it had three (3) layer in the rust as hematite (Fe O
O ) based for the outer layer, magnetite (Fe
) based for the outer layer, magnetite (Fe O
O ) based for the intermediate layer and Ni based layer for the inner layer. These steels showed mostly same Tafel gradient in their cathodic polarization curves compare with that for no rust specimens. However, the exchange current density which reaction is assumed as a hydrogen generation reaction was largely increased. The cathodic reaction for each steels whose surface is covered by magnetite layer might be accelerated, then the corrosion rate was considered as accelerated, too.
) based for the intermediate layer and Ni based layer for the inner layer. These steels showed mostly same Tafel gradient in their cathodic polarization curves compare with that for no rust specimens. However, the exchange current density which reaction is assumed as a hydrogen generation reaction was largely increased. The cathodic reaction for each steels whose surface is covered by magnetite layer might be accelerated, then the corrosion rate was considered as accelerated, too.
Akashi, Masatsune*; Fukaya, Yuichi*; Asano, Hidekazu*
JNC TJ8400 2000-014, 22 Pages, 2000/02
Difference of hydrogen generation phenomena on the surface of the Steels were not observed between carbon steel, atmospheric corrosion resisting steel and 5%-Ni steel. Rust layer was formed on these three-type of steels by steam oxidation method. And the chemical composition of the rust for the steels were basically two(2) layers structure for the previous two steels as hematite(Fe O
O ) based for the outer layer and magnetite(Fe
) based for the outer layer and magnetite(Fe O
O ) based for the inner layer. And for the last steel, it had three(3) layer in the rust as hematite(Fe
) based for the inner layer. And for the last steel, it had three(3) layer in the rust as hematite(Fe O
O ) based for the outer layer, magnetite(Fe
) based for the outer layer, magnetite(Fe O
O ) based for the intermediate layer and Ni based layer for the inner layer. These steels showed mostly same Tafel gradient in their cathodic polarization curves compare with that for no rust specimens. However, the exchange current density which reaction is assumed as a hydrogen generation reaction was largely increased. The cathodic reaction for each steels whose surface is covered by magnetite layer might be accelerated, then the corrosion rate was considered as accelerated, too.
) based for the intermediate layer and Ni based layer for the inner layer. These steels showed mostly same Tafel gradient in their cathodic polarization curves compare with that for no rust specimens. However, the exchange current density which reaction is assumed as a hydrogen generation reaction was largely increased. The cathodic reaction for each steels whose surface is covered by magnetite layer might be accelerated, then the corrosion rate was considered as accelerated, too.
Shibata, Toshio*; *; *; Tsuru, Toru*; Inoue, Hiroyuki*
JNC TJ8400 2000-013, 38 Pages, 2000/02
None
*; Futakawa, Masatoshi; Ioka, Ikuo; Onuki, Kaoru; Shimizu, Saburo; Eto, Motokuni; Oku, Tatsuo*; *
Zairyo, 48(7), p.746 - 752, 1999/07
no abstracts in English
; ; ; ; ; Takayanagi, Masaji
JAERI-Tech 94-016, 74 Pages, 1994/09
no abstracts in English
*
PNC TJ9605 91-001, 28 Pages, 1990/10
The present report describes the results of studies performed sa a part of the results of "CORROSION TESTS OF CLADDING INNER SURFACE COATING" during a period of Feb. 20 - Mar.30, 1990. In the present study, corrosion tests have been carried out with CsOH to evaluate corrosion resistance of ferritic stainless steel and coated stainless steel. The following results were drawn from the present study (1)Corrosion tests of austenitic and ferritic stainless steel with ScOH were made at temperatures of 500-700 C. After corrosin tests, intergranular attack was found to occur in the austenitic steel, however, there was no intergranular attack in the ferritic steel. Ferritic steel appears to have corrosion resistance to liquid CsOH superior to austenitic steel. (2)Corrosion tests between Ni-Ti, Ti, Al coatings on stainless steel and CsOH were made at temperature of 500-700
C. After corrosin tests, intergranular attack was found to occur in the austenitic steel, however, there was no intergranular attack in the ferritic steel. Ferritic steel appears to have corrosion resistance to liquid CsOH superior to austenitic steel. (2)Corrosion tests between Ni-Ti, Ti, Al coatings on stainless steel and CsOH were made at temperature of 500-700 C. Ni-Ti and Al coated stainless steel showed no intergranular attack, though the coatings were locally detached from the stainless stell substrates. Intergranular attack was observed in the Ti coated stainless steel. Ni-Ti and Al coating seem to be useful for reduction of intergranular attack of stainless steel cladding.
C. Ni-Ti and Al coated stainless steel showed no intergranular attack, though the coatings were locally detached from the stainless stell substrates. Intergranular attack was observed in the Ti coated stainless steel. Ni-Ti and Al coating seem to be useful for reduction of intergranular attack of stainless steel cladding.
; ; Nekoya, Shinichi; ;
JAERI-M 85-070, 34 Pages, 1985/06
no abstracts in English
; ;
Fushoku To Taisaku Jireishu, p.226 - 232, 1985/00
no abstracts in English
*; ;
Boshoku Gijutsu, 31(7), p.460 - 466, 1982/00
no abstracts in English
Boshoku Gijutsu, 11(8), p.347 - 350, 1962/00
no abstracts in English
 on crevice corrosion of type 316L stainless steel
on crevice corrosion of type 316L stainless steelAoyama, Takahito; Kato, Chiaki
no journal, ,
Alloyed Cu is known to inhibit the growth of pitting corrosion on stainless steels after pitting initiation. The Cu dissolved from the stainless steel matrix acts as an inhibitor in acidic chloride environments which is formed in pits. The Cu
dissolved from the stainless steel matrix acts as an inhibitor in acidic chloride environments which is formed in pits. The Cu suppresses active dissolution rate inside the pits
suppresses active dissolution rate inside the pits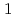 . This suppression effect is supposed to be also effective for active dissolution which promotes crevice corrosion. Therefore, if it is possible to introduce Cu
. This suppression effect is supposed to be also effective for active dissolution which promotes crevice corrosion. Therefore, if it is possible to introduce Cu to the inside of a crevice from the outside, introduced Cu
to the inside of a crevice from the outside, introduced Cu is supposed to inhibit crevice corrosion on stainless steel. However, diffusion of ions between inside and outside of the crevice is restricted by its geometry, and Cu
is supposed to inhibit crevice corrosion on stainless steel. However, diffusion of ions between inside and outside of the crevice is restricted by its geometry, and Cu does not migrate to inside of a crevice according to the electroneutrality principle. [Cu (EDTA (ethylenediaminetetraacetic acid))]
does not migrate to inside of a crevice according to the electroneutrality principle. [Cu (EDTA (ethylenediaminetetraacetic acid))] is a chelated Cu
is a chelated Cu which has negative charge, and is expected to migrate to inside of a crevice from the outside by electrochemical migration. In addition, it is reported that Cu
which has negative charge, and is expected to migrate to inside of a crevice from the outside by electrochemical migration. In addition, it is reported that Cu in [Cu(EDTA)]
in [Cu(EDTA)] could be easily substituted by Fe
could be easily substituted by Fe in low pH
in low pH . Therefore, Cu
. Therefore, Cu is considered to be introduced to the inside of the crevice as [Cu(EDTA)]
is considered to be introduced to the inside of the crevice as [Cu(EDTA)] , and to affect crevice corrosion of stainless steel. In this study, in situ observation of an inside of the crevice
, and to affect crevice corrosion of stainless steel. In this study, in situ observation of an inside of the crevice was performed to analyze the effect of [Cu(EDTA)]
was performed to analyze the effect of [Cu(EDTA)] on crevice corrosion.
on crevice corrosion.
Suda, Shoya; Masai, Seita; Kawahara, Takahiro; Fujikura, Toshiki; Hoshi, Akiko; Wakai, Eiichi; Kondo, Keietsu; Nishimura, Akihiko; Minehara, Eisuke*
no journal, ,
no abstracts in English
大谷 恭平; 加藤 千明
not registered
【課題】本発明は、添加コストや排水の水処理コストが低い防食剤および防食方法を提供することを課題とする。 【解決手段】本発明は、(A)乳酸アルミニウム、および(B)無機酸塩および有機酸塩から選ばれる1種または2種類以上を含有する、水系における金属部材の防食剤、および同防食剤を用いた水系における金属部材の防食方法を提供する。